Theme: Discoveries and Developments for Safer Medication
Drug Monitoring 2016
We welcome you to the 2nd International Conference on Clinical Trials and Therapeutic Drug Monitoring which is scheduled to be held during August 22-24, 2016 at Philadelphia, USA.
Drug monitoring conferences has theme as “Discoveries and Developments for Safer Medications”. The focus will be mainly on the Therapeutic Drug Monitoring and Toxicogenomics, factors influencing them, challenges, methods followed, rapidly-emerging techniques and their applications.Therapeutic drug monitoring means concentrations of drugs in body fluids, usually plasma, this can be used for diagnostic purposes and during treatment. The selection of drugs for therapeutic drug monitoring is important as the effects of many drugs is not clearly related to their concentrations. Therapeutic drug monitoring is the clinical practice of measuring concentration of specific drugs in a patient's bloodstream at designated intervals to maintain a constant concentration, thereby optimizing individual dosage regimens. Quantification of Drugs using the analytical methods helps in predicting the toxicological effects.
Therapeutic drug monitoring is defined as the clinical laboratory measurement of a chemical parameter that will directly influence drug prescribing procedures after appropriate medical interpretation. TDM refers to maintaining blood drug or plasma concentrations within a targeted therapeutic range or window for the individualization of drug dosage. Drug Monitoring begins after the drug is first prescribed, and involves determining an initial dosage regimen appropriate for the clinical condition and patient characteristics like age, organ function, weight and concomitant drug therapy.
Related Pharmaceutical Conferences | Drug Monitoring Conferences | Drug Delivery Conferences
8th International Conference on Novel Drug Delivery Systems, March 07-09, 2016, Spain; 2nd Drug Discovery Conference & Designing, October 24-26, 2016, Turkey ; 4th International Computer Aided Drug Designing Conference, November 02-04, 2015, USA; 7th Annual Global Pharma Summit, June 20-22, 2016, USA ; 9th World Drug Delivery Summit, June 30-July 02, 2016, USA ; 14th Therapeutic Drug monitoring and Clinical Toxicology Conference, October 11-15, Netherlands, Midwest Association for Toxicology and Therapeutic Drug Monitoring Conferences, April 14-15, 2016, USA; 9th Annual Pain & Migraine Therapeutics Summit, September 23-24, 2015 USA, Americas Drug Safety Congress, April 20-21, 2016, USA, 2nd Annual Formulation & Drug Monitoring USA Congress, May 18-19, 2016, USA; ANZ Therapeutic Drug Monitoring Workshop; MATT : Midwest Association for Toxicology and Therapeutic Drug Monitoring; IATDMCT: International Association of Therapeutic Drug Monitoring and Clinical Toxicology; SOT: Society of Toxicology; BTS: British Toxicology Society
Pharmacokinetics & Pharmacodynamics
Pharmacokinetics involves the measurement of the time course of drug absorption, distribution, metabolism and excretion. Clinical pharmacokinetics means the application of pharmacokinetic principles to the safe and effective therapeutic management of drugs in an individual patient. Pharmacodynamics means the relationship between drug concentration at the site of action and the resulting effect, intensity of therapeutic and adverse effects and including the time course. Therapeutic treatment requires information on both pharmacokinetics and pharmacodynamics to understand interpatient variability and how to prescribe dosage for body size, age, or genetic traits. Clinical pharmacokinetic drug trials when compiled for numerous drugs can reveal metabolism and excretion differences across age groups. If sufficient clinical pharmacokinetic data is available, it may be possible to make inferences about the handling of environmental toxicants in a particular age group.
Related Pharmaceutical Conferences | Pharmacokinetic Conferences | Clinical Pharmacy Conferences |
7th Annual Global Pharma Summit, June 20-22, 2016, USA; World Pharma Congress, November 07-November 09, 2016, USA; Pharmaceutical Summit and Expo October 08-10, 2015, India; 6th Asia-Pacific Pharma Congress July 11-13, 2016, Malaysia; 8th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Conference, March 07-09, 2016, Spain; 2nd Annual Drug Discovery Congress, October 29-30,2015, USA, Drug Discovery Congress 2016, Boston, USA; 10th Drug Design and Medicinal Chemistry, May 09-10, 2016, Germany; Drug Discovery week Europe, October 05-08, 2015, Germany; 3rd Antibody & Protein Therapeutics, October 22-23, 2015, USA; 4th Immunogenicity & Immunotoxicity, January 25-26,2016, USA; IATDMCT: International Association of Therapeutic Drug Monitoring and Clinical Toxicology; ACCP: American College of Clinical Pharmacy; UKCPA: United Kingdom Clinical Pharmacy Association; ESCP: The European Society of Clinical Pharmacy; Royal Pharmaceutical Society
The ADME of a drug determines the Serum Concentration of a particular drug. Serum drug concentration is mainly affected by characteristics like genetic makeup, pathophysiology condition and various other factors. The metabolism of drugs in patient with renal failure may slowly clears a drug in urine than a patient with normal renal function. Pregnancy state in women alters the drug metabolism and drug-drug interactions may also alter serum drug concentration. Anatomical variations and different metabolism rates of specific drugs in men and women may show both pharmacokinetic and pharmacodynamics differences.
Related Toxicology Conferences | Pathology Conferences | Drug Monitoring Conferences
5th International Pathology Conference May 09-11, 2016, USA; World Digital Pathology Congress October 31-November 02, 2016, Spain; International Internal Medicine Conference November 03-05, 2016, USA; International Conference on Speech-Language Pathology November 14-15, 2016 Atlanta, USA; International Conference on Cytology & Histology August 01-03, 2016, UK; Pathology Summit Update 2016, February 26-28, 2016, Australia; American society for Clinical Pathology Annual Meeting, October 28-30, 2015, USA; 29th Annual Park city Anatomic Pathology Update, February 7-11, 2016, USA, 10th Drug Design and Medicinal Chemistry, May 09-10,2016, Germany; European Pharma Summit, May 09-11, 2016, Germany; ESCP: The European Society of Clinical Pharmacy; Pharmaceutical Society of Australia; National Pharmacy Association; ACCP: American College of Clinical Pharmacy; American Society for Clinical Pharmacology and Therapeutics
Anti-epileptic drugs have a narrow therapeutic range as they are strongly bound to serum proteins. Cardio active drugs Digoxin which is frequently used has a narrow therapeutic window. The immunoassays employed in monitoring concentration of serum digoxin are subjected to interference from both exogenous and endogenous compounds. Anti-asthmatic drugs like theophylline and caffeine also require Therapeutic Drug Monitoring. Antidepressants monitoring is essential to avoid drug toxicity. Immunosuppressant’s Whole blood concentration is determined to avoid drug toxicity as well as subtherapeutic levels which may lead to organ rejection. Antibiotics like aminoglycoside produce Nephrotoxicity and Ototoxicity for which they are monitored.
Related Toxicology Conferences | Pharmaceutical Conferences | Drug Discovery Conferences
4th Annual conferences on European Pharma Congress, June 13-15, 2016, Germany; 2nd International Conference on Drug Discovery & Designing, October 24-26, 2016, Turkey; 9th World Drug Delivery Summit, June 30-July 02, 2016, USA; 8th International Conference on Novel Drug Delivery Systems, March 07-09, 2016, Spain; 7th Annual Global Pharma Summit June 20-22, 2016, USA; 18th International Conference on Toxicogenomics Conferences, Drug Monitoring and Toxicology, February 1-2, 2016, Brazil; 9th Annual Pain & Migraine Toxicogenomics 2016 Summit, September 23-24, 2015 USA, World Drug Monitoring USA, April 20-21, 2016, USA, 2nd Annual Formulation & Drug Delivery Congress, May 18-19, 2016, UK; European Pharma Summit, May 09-11, 2016, Germany; SASOCP: South African Society of Clinical Pharmacy; American Society of Clinical Pharmacists; EAHP: European Association of Hosiptal Pharmacists; American College of Toxicology; ANZ Therapeutic Drug Monitoring Workshop
Drug assay methods should have adequately sensitivity. They should be specific to the drug metabolites which has to be measured and should be accurate and precise. Most of the high-volume drug assays are now performed with the automated immunoassay methods which possess these characteristics. However, many a number continue to require manual assay procedures like HPLC and GLC (e.g. amiodarone, perhexiline). Depending on the equipment used, plasma or serum are usually used for drug assays.
Related Analysis Conferences | Toxicology Conferences | Pharmacological Conferences
7th International Conference on Analytical & Bioanalytical Techniques, September 29-October 01, 2016, USA; 2nd International Conference on Separation Techniques, November 03-05, 2016, Spain; International Conference on Biopharmaceutics, September 21-22, 2015, USA; 2nd International Mass Spectrometry Conference, May 09-11, 2016, USA; 4th Annual conferences on European Pharma Congress, June 13-15, 2016, Germany; 2016 Winter Conference on Plasma Spectrochemistry, January 10-16, 2016, USA; International Forum Process Analysis & Control, January 24-27, 2016, USA; Structure Based Drug Monitoring USA Conference 2016, February 21-24, 2016, USA, 1st International Conference on Applied Chemistry, March 14-17, 2016, Egypt; Experimental Nuclear Magnetic Resonance Conference 2016, April 10-15, USA; DATIA: Drug and Alcohol Testing Industry Association; National Pharmacy Association; Royal Pharmaceutical Socitey; ANZ Therapeutic Drug Monitoring Workshop; MATT : Midwest Association for Toxicology and Therapeutic Drug Monitoring;
Therapeutic Drug Monitoring depends on Chemical Analysis, Pharmacokinetics and Pharmacodynamics. By determining the nature of the problem to be solved, appropriate methodology to solve the problem, developing applicable analytical schemes that have to be performed competently with good quality and interpreted within the framework, analytical goals in therapeutic drug monitoring should be established. If plasma drug concentration measurements are to be of any particular value, blood sampling timing, the type of blood sample, the measurement technique, and the interpretation of results are to be done with attention. After dosing, it is crucial to obtain the blood sample for measuring the drug concentration at the correct time. Errors in the sampling times are likely responsible for the errors in interpreting the results.
Related Toxicology Conferences | Pharmaceutical Conferences | Genomic Conferences
4th Annual conferences on European Pharma Congress, June 13-15, 2016, Germany; World Pharma Congress, October 31-November 02, 2016, USA; Pharmaceutical Summit and Expo, October 08-10, 2015, India; 7th International Conference on Analytical & Bioanalytical Techniques, September 29-October 01, 2016 Miami, USA; 8th International Conference on Pharmaceutics & Novel Drug Delivery Systems, March 07-09, 2016, Spain; 14th Therapeutic Drug Monitoring Confernces and Clinical Toxicology Conference, October 11-15, Netherlands, Midwest Association for Toxicology and Therapeutic Drug Monitoring, April 14-15, 2016, USA; 17th Annual Toxicogenomics 2016 Summit, June 2016, Germany; 2nd Annual Formulation & Drug Delivery Congress, May 18-19, UK; European Pharma Summit, May 09-11, 2016, Germany; EAHP: European Association of Hosiptal Pharmacists; American College of Toxicology; ANZ Therapeutic Drug Monitoring Workshop; ESCP: The European Society of Clinical Pharmacy; Pharmaceutical Society of Australia; National Pharmacy Association
Clinician with good insight into the factors determining the patient’s response to drug therapy can use drug monitoring data. In certain conditions were a patient fails to respond to a usual therapeutic dose, measurement of plasma level can help to determine who is a true non-responder and who is a noncompliant patient. Therapeutic Drug Monitoring also provides useful information regarding individual changes in drug utilization patterns. Drug Monitoring also provides data in changes in utilization of drug as a consequence of disease process or altered physiological state. TDM is a useful adjunct in treating many patients provided the potential pit falls.
Related Human Genetics Conferences | Toxicology Conferences | Environmental Toxicology Conferences
7th International Analytical Conference, September 29-October 01, 2016, USA; International Conference on Drug Discovery & Designing August 11-13, 2015 Frankfurt, Germany; 6th Asia Pacific Pharma Congress July 11-13, 2016, Malaysia; 8th International Conference on Novel Drug Delivery Systems, March 07-09, 2016, Spain; 14th Therapeutic Drug monitoring Conferences and Clinical Toxicology Conference, October 11-15, Netherlands, Midwest Association for Toxicogenomics USA and Therapeutic Drug Monitoring, April 14-15, 2016, USA; 17th Annual Drug Discovery Summit, June 2016, Germany; 2nd Annual Formulation & Drug Monitoring USA Congress, May 18-19, USA; European Pharma Summit, May 09-11, 2016, Germany; SASOCP: South African Society of Clinical Pharmacy; American Society of Clinical Pharmacists; EAHP: European Association of Hosiptal Pharmacists; American College of Toxicology; ANZ Therapeutic Drug Monitoring Workshop
Toxicity detection requires a means to observe (or measure) specific effects of exposures. Toxicology is focused on changes in an organism as a result of exposure to physical, chemical, or biologic agents. Such changes range from reversible effects like transient skin reactions and chronic diseases like as cancer, or to the extreme end point of death. Studies of whole-animal toxicology may range from single-dose acute to chronic lifetime exposures and they include determination of end points like clinical signs of toxicity, organ and body weight changes, clinical chemistry, and histopathologic responses. Predictive toxicology means the study of how toxic effects noted in humans or model systems can be used to assess risk, predict pathogenesis and prevent human disease. Predictive toxicology not only includes risk assessment but also the practical facilitation of decision making with scientific information.
Related Toxicology Conferences | Pharmacology Conferences | Genomics Conferences
5th Global Toxicology Summit & Applied Pharmacology, September 19-21, 2016, USA; 6th Global Toxicology & Applied Pharmacology Conference , October 27-29, 2016, Italy; 2nd International Conference on Pharmacology and Ethnopharmacology, May 02-04, 2016, USA; International Environmental Toxicology Conference and Ecological Risk Assessment, August 25-26, 2016, Brazil; 5th Global Toxicology Summit & Applied Pharmacology, September 19-21, 2016, USA; 7th Society of Environmental Toxicology and Chemistry, October 4-8, 2015, South Africa, Society of Forensic Toxicology, October 18-23, 2015, USA; Environmental Mutagenesis and Genomics Society, September 26-30, USA; European Congress of the European Societies of Toxicogenomics 2016, September 04-07, 2016, Turkey; Epigenomics & Novel Therapeutic Targets, May 26-27, 2016, USA; 18th International Conference on Toxicogenomics Conferences, Drug Monitoring and Toxicology, February 1-2, 2016; SOT: Society of Toxicology; BTS: British Toxicology Society; Eurotox: Federation of European Toxicologists & European Societies of Toxicology; IUTOX: International Union of Toxicology; ESTIV: European Society of Toxicology In Vitro
Toxicogenomic technologies comprises of several different technology platforms which are used for analysis of genomes, proteins, metabolites and transcripts. Genomic technologies includes genome sequencing technologies to derive DNA sequences from genes and other parts of DNA and genotype analysis, to detect sequence variations between individual genes. Transcriptomic technologies measures mRNA expression in a highly parallel assay system, mostly using microarrays. Proteomics studies is the collections of proteins in living systems as the same proteins may be present in multiple modified and variant forms. Metabolomics means the study of small-molecule components of biologic systems.As metabolites reflect the activities of proteins, RNAs and the genes that encode them. Metabolomics provides the functional assessment of diseases and chemical and drug toxicity. Bioinformatics focuses on applying advanced computational techniques to the biologic data and to perform all the genomic technologies.
Related Toxicology Conferences | Genomics Conferences | Metabolomics Conferences
5th Global Toxicology Summit & Applied Pharmacology, September 19-21, 2016, USA; 6th Global Summit on Toxicology & Applied Pharmacology Conferences, October 27-29, 2016, Italy; 2nd International Conference on Pharmacology and Ethnopharmacology, May 02-04, 2016, USA; International Conference on Environmental Toxicology and Ecological Risk Assessment, August 25-26, 2016, Brazil; International Conference on Neuro Genetics, June 06-07, 2016, UK; Environmental Mutagenesis and Toxicogenomics USA Conferences Society, September 26-30, USA; European Congress of the European Societies of Toxicology 2016, September 04-07, 2016, Turkey, Society of Toxicology of Canada, December 08-10, 2015, Canada, 8th International Toxicology Conference on , 2018, Thailand,17th International Toxicology Conference, September 25-26, 2015 UK; 18th International Conference on Toxicogenomics Conferences, Drug Monitoring and Toxicology, February 1-2, 2016; American College of Toxicology; American Society for Clinical Pharmacology and Therapeutics; ASIATOX: The Asian Society of Toxicology; IATDMCT: International Association of Therapeutic Drug Monitoring and Clinical Toxicology; MATT: Midwest Association for Toxicology and Therapeutic Drug Monitoring
An adverse outcome pathway (AOP) is a conceptual framework that organizes existing knowledge concerning biologically plausible, and empirically supported, links between molecular-level perturbation of a biological system and an adverse outcomes at a level of biological organization of regulatory relevance. Systematic organization of information into AOP frameworks has potential to improve regulatory decision-making through greater integration and more meaningful use of mechanistic data. However, for the scientific community to collectively develop a useful AOP knowledgebase that encompasses toxicological contexts of concern to human health and ecological risk assessment, it is critical that AOPs be developed in accordance with a consistent set of core principles. Based on the experiences and scientific discourse among a group of AOP practitioners, we propose a set of five fundamental principles that guide AOP development: (1) AOPs are not chemical specific; (2) AOPs are modular and composed of reusable components-notably key events (KEs) and key event relationships (KERs); (3) an individual AOP, composed of a single sequence of KEs and KERs, is a pragmatic unit of AOP development and evaluation; (4) networks composed of multiple AOPs that share common KEs and KERs are likely to be the functional unit of prediction for most real-world scenarios; and (5) AOPs are living documents that will evolve over time as new knowledge is generated. The goal of the present article was to introduce some strategies for AOP development and detail the rationale behind these 5 key principles. Consideration of these principles addresses many of the current uncertainties regarding the AOP framework and its application and is intended to foster greater consistency in adverse reactions development.
Related Toxicology Conferences | Pharmacology Conferences | Adverse Drug Reaction Conferences
5th Global Toxicology Summit & Applied Pharmacology, September 19-21, 2016, USA; 6th Global Toxicology Summit & Applied Pharmacology, October 27-29, 2016, Italy; 2nd International Conference on Pharmacology and Ethnopharmacology, May 02-04, 2016, USA; International Conference on Environmental Toxicology and Ecological Risk Assessment, August 25-26, 2016, Brazil; International Conference on Neuro Genetics, June 06-07, 2016, UK; Asia Pacific Association of Medical Toxicology, December 1-4, 2015, Western Australia; 7th Society of Environmental Toxicology and Chemistry, October 4-8, 2015, South Africa, Society of Toxicogenomics USA, October 18-23, 2015, USA; Environmental Mutagenesis and Genomics Society, September 26-30, USA; European Congress of the European Societies of Toxicogenomics 2016, September 04-07, 2016, Turkey; ASIATOX: The Asian Society of Toxicology; IATDMCT: International Association of Therapeutic Drug Monitoring and Clinical Toxicology; MATT: Midwest Association for Toxicology and Therapeutic Drug Monitoring; SOT: Society of Toxicology; BTS: British Toxicology Society;
Environmental genomics has revolutionized the study of adverse effects of environmental toxicants. It gives an efficient and high-throughput means to understand the mechanisms of action, risk assessment and to identify and understand basic pathogenic mechanisms that are crucial in disease progression, predicting toxicity and to more precisely phenotype disease subtypes. Environmental genomics in a toxicant exposure model is a crucial tool in biological response marker or biomarker discovery.
Related Environmental Toxicology Conferences | Toxicology Conferences | Toxicogenomics Conferences
International Conference on Environmental Toxicology and Ecological Risk Assessment, August 25-26, 2016, Brazil; 5th Global Toxicology Conference & Applied Pharmacology, September 19-21, 2016, USA; 6th Global Summit on Toxicology & Applied Pharmacology, October 27-29, 2016, Italy; International Conference on Ethnopharmacology, May 02-04, 2016, USA; 6th Asia Pacific Pharma Congress July 11-13, 2016, Malaysia; Biomarker Summit Europe 2015, October 05-08, 2015, Germany; 9th Biomarkers in Drug Discovery & Development, October 05-06, 2015, Germany; 4th Biomarkers in Diagnostics, October 07-08, 2015, Germany; European Pharma Summit, May 09-11, 2015, Germany; Epigenomics & Novel Therapeutic Targets, May 26-27, 2016, USA; 18th International Conference on Toxicogenomics Conferences, Drug Monitoring and Toxicology, February 1-2, 2016; American College of Toxicology; American Society for Clinical Pharmacology and Therapeutics; ASIATOX: The Asian Society of Toxicology; IATDMCT: International Association of Therapeutic Drug Monitoring and Clinical Toxicology; MATT: Midwest Association for Toxicology and Therapeutic Drug Monitoring
Comparative Toxicogenomics is a research tool that curates scientific data describing relationships between chemicals, drugs and genes, proteins, GO annotations, pathways, diseases, taxa, phenotypes, and interaction modules. Comparative Toxicogenomics gives information on Gene-disease associations, Chemical-disease associations, Chemical-gene interactions and Chemical-phenotype associations.
Related Toxicology Conferences | Toxicogenomics Conferences | Genetic Conferences
International Conference on Environmental Toxicology and Ecological Risk Assessment, August 25-26, 2016, Brazil; 5th Global Summit on Toxicology & Applied Pharmacology, September 19-21, 2016, USA; 6th Global Summit on Toxicology & Applied Pharmacology, October 27-29, 2016, Italy; International Conference on Pharmacology and Ethnopharmacology, May 02-04, 2016, USA; 6th Asia Pacific Pharma Congress July 11-13, 2016, Malaysia; Sequencing & Bioinformatics, October 05-07, 2015, Germany; Protein Discovery Summit, October 23-24, 2015, USA; Single Cell Genomics Conferences & Transcriptomics USA Congress, October 27-28, 2015,USA; American College of Toxicogenomics USA, November 08-11, 2015, USA; The American Academy of Clinical Toxicology, October 08-09, 2015, USA; IATDMCT: International Association of Therapeutic Drug Monitoring and Clinical Toxicology; MATT: Midwest Association for Toxicology and Therapeutic Drug Monitoring; SOT: Society of Toxicology; IUTOX: International Union of Toxicology; ESTIV: European Society of Toxicology In Vitro
Toxicogenomics challenges include Gene annotation, Cross-species extrapolation, Technical standards for evolving platforms, Standards for data sharing, Biomarker qualification and Translation of assays for regulatory purposes.
Related Toxicology Conferences | Toxicogenomics Conferences | Pharmaceutical Conferences
3rd International Conference on Genomics & Pharmacogenomics, September 21-23, 2015, USA; World Congress on Human Genetics, October 31- November 02, 2016, Spain; International Conference on Genomic Medicine, August 11-12, 2016, UK; 5th Global Summit on Toxicology & Applied Pharmacology, September 19-21, 2016, USA; 6th Global Summit on Toxicology & Applied Pharmacology, October 27-29, 2016, Italy; European Pharma Summit, May 09-11, 2016, Germany; Epigenomics & Novel Therapeutic Targets, Boston, USA, Genome Editing Congress, August 27-28, 2016, UK; Sequencing & Bioinformatics, October 05-07, 2015, Germany; Protein Discovery Summit, October 22-23, 2015, USA; 18th International Conference on Toxicogenomics Conferences, Drug Monitoring and Toxicology, February 1-2, 2016; MATT: Midwest Association for Toxicology and Therapeutic Drug Monitoring; SOT: Society of Toxicology; BTS: British Toxicology Society; National Pharmacy Association; American Society for Clinical Pharmacology and Therapeutics
As toxicogenomics continues to move forward, it will likely seem at times as if progression is standing still and at other times advancing quickly. In order to advance, key milestones will require coordination across fields and disciplines, so progress is likely to be incremental. Within the next 5 years, it is likely that toxicogenomics will move slowly forward. Biologists will continue to impact the field of informatics, and what constitutes a pathway will be better defined and begun to be standardized universally. In addition, the work of discovery and validation of prodromal biomarkers for a variety of toxicities and diseases will continue to evolve. The advance in technology will no doubt come with reduced cost per sample for analysis and will enable simultaneous probing of genetic, genomic, proteomic and metabolomic events. In the regulatory environment, toxicogenomics biomarker data will routinely be used to better inform the risk assessment from in vitro and in vivo test systems. The acceptance of modified test systems will eventually lead to an impact that minimizes animal testing and allows efficient modelling from human in vitro–based assays and ultra lowdose testing of human subjects to extrapolate and inform toxicity predictions. These models will eventually lead way to predictive in silico models that can help reduce use of animals and cost of experiments conducted to assess hazard and risk.
Related Toxicology Conferences | Proteomics Conferences | Genomics Conferences
World Congress on Human Genetics, October 31- November 02, 2016, Spain; International Conference on Genomic Medicine August 11-12, 2016 Birmingham, UK; 3rd International Conference on Genomics & Pharmacogenomics, September 21-23, 2015, USA; Conference on Genetic Counseling and Genomic Medicine, August 11-12, 2016, UK; 5th Global Summit on Toxicology & Applied Pharmacology, September 19-21, 2016, USA; 17th International Conference on Toxicology, September 25-26, 2015, UK, Genetic Toxicology Association Meeting, May 05-06, USA; Canadian Ecotoxicity Workshop, October 04-07, 2015, Canada; Epigenomics & Novel Therapeutic Targets, Boston, USA, Genome Editing Congress, August 27-28, 2016, UK; European Congress of the European Societies of Toxicogenomics USA 2016, September 04-07, 2016, USA; SOT: Society of Toxicology; BTS: British Toxicology Society; Eurotox: Federation of European Toxicologists & European Societies of Toxicology; IUTOX: International Union of Toxicology; ESTIV: European Society of Toxicology In Vitro
We are pleased to organize 2nd International Conferfence on Clinical Trials and Therapeutic Drug Monitoring during August 22-24, 2016 at Philadelphia, USA.The Conference mainly focuses on the theme "Discoveries and Developments for Safer Medication".It is an extraordinary event designed for the professionals to facilitate the dissemination and application of research finding on Drug Monitoring and Toxicogenomics.
Drug Monitoring 2016 is a multidisciplinary approach. Drug concentration measurements are requested to assist the management of a patient's current medication regimen or to screen for a medicine. The Drug Monitoring studies are performed for monitoring drugs with narrow therapeutic ranges, drugs with marked pharmacokinetic variability, medications for which target concentrations are difficult to monitor, and drugs known to cause therapeutic and adverse effects. The process of TDM is predicated on the assumption that there is a definable relationship between dose and plasma or blood drug concentration, and between concentration and therapeutic effects. Accurate and clinically meaningful drug concentrations are attainable only by complete collaboration by a TDM team, typically comprised of scientists, clinicians, nurses, and pharmacists. OMICS International organizes a conference series of 1000+ Global Events inclusive of 300+ Conferences, 500+ Upcoming and Previous Symposiums and Workshops in USA, Europe & Asia with support from 1000 more scientific societies and publishes 700+ Open access journals which contains over 30000 eminent personalities, reputed scientists as editorial board members.
Why to Attend??
Drug Monitoring 2016 gives you access to leading-edge pharmaceutical information and valuable new professional contact from all over the world. Its large international participation provides excellent opportunities for global sharing of technology, expertise, products, and best practices. There are exhibitions which run in parallel with the conference and offer you the chance to get up-to-date information from companies active in your area of interests. We are grateful to our sponsors and exhibitors for their interest and support for the conference.
Target Audience:
- Professors, Associate Professors, Assistant Professors
- PhD Scholars
- Graduates and Post Graduates
- Directors, CEO’s of Organizations
- Association, Association presidents and professionals
- Noble laureates in Health Care and Medicine
- Pharmacists
- Toxicology Professionals
- Genetic Professionals
- Bio instruments Professionals
- Bio-informatics Professionals
- Software development companies
- Research Institutes and members
- Supply Chain companies
- Manufacturing Companies
- Training Institutes
- Business Entrepreneur
Summary:
OMICS International Conferences invites all the participants across the globe to attend 2nd International Conference on Clinical Trials and Therapeutic Drug Monitoring during August 22-24, 2016 in Philadelphia, USA which includes prompt keynote presentations, Oral talks, Poster presentations and Exhibitions. Drug Monitoring 2016 is a specially designed cluster conference. The main theme of the conference is “Discoveries and Developments for Safer Medication” which covers a wide range of critically important sessions.
For More details please visit: http://toxicogenomics.conferenceseries.com/
Importance and Scope:
Therapeutic drug monitoring, a multi-disciplinary clinical specialty, aimed at improving patient care by monitoring drug levels in the blood to individually adjust the dose of drugs for improving outcome. TDM is viewed as a component of personalized medicine that interacts with several other disciplines including pharmacokinetics and pharmacogenetics. One chapter is devoted to monitoring of drugs of abuse.
Why Philadelphia, USA?
Philadelphia is the largest city in the Commonwealth of Pennsylvania. Widely known as Philly, but formerly known as the City of Philadelphia. It is also called the City of Brotherly Love. The fifth most populous city in the United States, and the core of the sixth-largest metropolitan area in the country. There are nearly 1.5 million people in the city alone. The city is located in the Northeastern United States at the confluence of the Delaware and Schuylkill rivers; Philadelphia is the economic and cultural center of the Delaware Valley.
The city has more outdoor sculptures and murals than any other American city, and Philadelphia’s Fairmount Park is the largest landscaped urban park in the world. The birthplace of America is Philadelphia, the city where the founding fathers lived and the Declaration of Independence was signed. Today that history echoes in the original buildings and museums of the area often called “Historic Philadelphia”. Lacking defined boundaries, Historic Philadelphia covers parts of the Old City and Center City neighborhoods and includes the Independence National Historic Park. Some of the top Historic Philadelphia attractions are not exhaustive, but it does include some of the most popular, interesting and informative places to visit in the area like City Center, Independence National Historical Park, Pennsylvania Academy of Fine Arts Museum, Rittenhouse, Reading Terminal Market, Please Touch Museum, Philadelphia Museum of Art, Franklin Square, Mutter Museum.
Therapeutic drug monitoring (TDM) will spike to $2.69 billion by 2019. Therapeutic drug monitoring can be categorized into seven main modalities: antiepileptics, antiarrhythmics, antibiotics, antineoplastics, bronchodilators, immunosuppressives and HIV/AIDS drugs. The global therapeutic drug monitoring market is dominated by eight major suppliers that account for a combined 88.3% of the global market. The U.S. market is expected to grow to $1.84 billion by 2019 at a CAGR of 12.6%.
Conference Highlights:
• Drug monitoring
• Pharmacokinetics & Pharmacodynamics
• Gender & Pathophysiology
• Individual Drugs TDM
• Drug Quantification
• TDM Challenges
• Advances in TDM
• Toxicogenomics Roles
• OMICS Technologies
• Adverse Outcome Pathways
• Environmental Toxicogenomics
• Comparative Toxicogenomics
• Toxicogenomics Challenges
• Toxicogenomics Applications
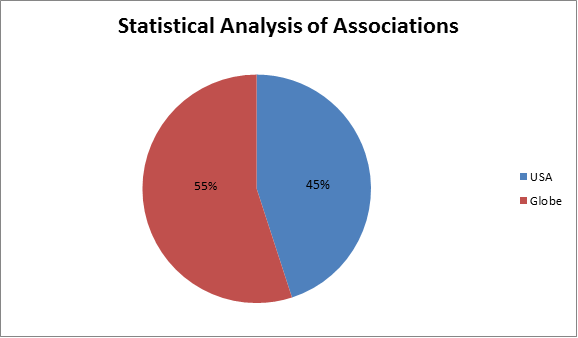
Major Associations around the Globe:
• International Association of Therapeutic Drug Monitoring and Clinical Toxicology
• Midwest Association for Toxicology and Therapeutic Drug Monitoring
• Academy of Managed Care Pharmacy (AMCP), Alexandria, VA
• Society of Toxicology (SOT)
• Drug Information Association
• British Columbia Pharmacy Association
• California Pharmacists Association
• Kansas Pharmacists Association
• Society of Toxicology of Canada
• Toxicology Education Foundation (TEF)
• International Society of Regulatory Toxicology and Pharmacology
Major Associations in USA:
• American Academy of Clinical Toxicology
• American Association for Laboratory Animal Science
• American Board of Toxicology
• American Board of Forensic Toxicology
• American Board of Veterinary Toxicology
• American Association of Pakistani Pharmaceutical Scientists
• American College of Toxicology
• Behavioral Toxicology Society
• Drug Information Association
• Ohio Pharmacists Association
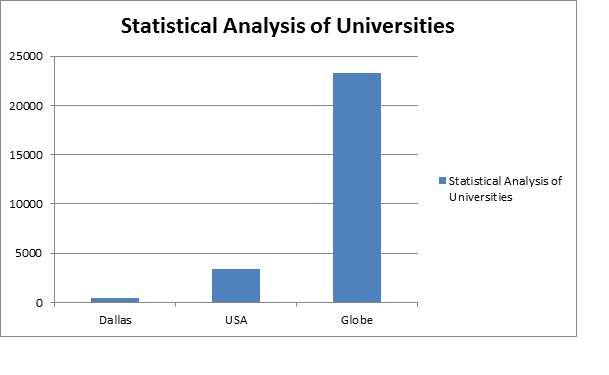
Top Universities in USA :
- Auburn University
- Samford University
- Midwestern University
- The University of Arizona
- Harding University
- University of Arkansas for Medical Sciences
- California Health Sciences University
- Keck Graduate Institute
- University of California, San Francisco
- Idaho State University
- University of Illinois at Chicago
- The University of Kansas
- Wayne State University
- University of Kentucky
- University of the Pacific
Target Audience:
- Professors, Associate Professors, Assistant Professors
- PhD Scholars
- Graduates and Post Graduates
- Directors, CEO’s of Organizations
- Association, Association presidents and professionals
- Noble laureates in Health Care and Medicine
- Pharmacists
- Toxicology Professionals
- Genetic Professionals
- Bio instruments Professionals
- Bio-informatics Professionals
- Software development companies
- Research Institutes and members
- Supply Chain companies
- Manufacturing Companies
- Training Institutes
- Business Entrepreneur
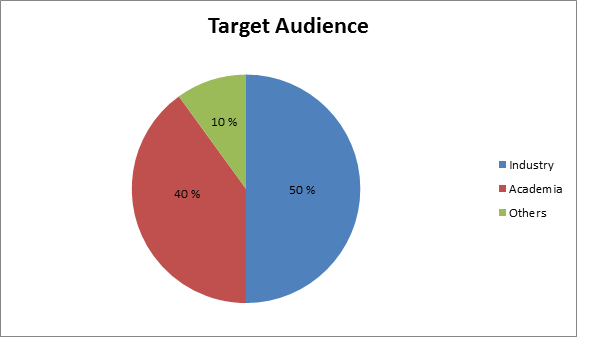
Target Audience:
- Industry 50%
- Academia 40%
- Others 10%
Glance at the Market:
The global in-vitro toxicology testing market is estimated to reach $17,227 million by 2018 at a CAGR of 13.5% during the forecast period (2013–2018). The market will witness a double-digit growth attributed to the increasing acceptance of in vitro methods over in vivo ones. Government support to stop animal testing, new and promising technologies, and advancement in new approaches are significant factors forcing the market in the forecast period.
Initiation of Tox 21, government programs by the U.S. government and growing number of drug discoveries and innovations globally represent an opportunity for the growth of the market. Detection of the toxic effects much earlier in the development stage, the pharmaceutical industry adapted to in vitro methods apart from the rising pressure to reduce the drug attrition rates to control the drug development costs and time line. The pharmaceutical industry has been witnessing a enormous growth as a result of government initiatives such as AXLR8 program that was initiated by the European Union. The cosmetic industry will grow the fastest owing to the amendment of European Union’s Cosmetics Directive that has set forth ban on the use of animals in testing for any toxic effects of beauty products. Geographic analysis reveals that Europe was the largest contributor to the global in vitro toxicology testing market in 2013. It will also be the fastest growing region till 2018. The European market is witnessing growth as a result of strong government directives to altogether stop animal testing and replace in vivo testing by in vitro methods.
Global in vitro Toxicology market

Conference Highlights
- Clinical Data Management and Statistics
- Clinical Research and Trials on Diabetes/ AIDS / Cancer
- Clinical Trials on different Diseases and Medical Devices
- Clinical Trials (Countries/Continents)
- CRO/ Sponsorship and Future of Clinical Trials
- Bioethics, Quality Regulation and Case Reports
- Clinical Trial Supply Management
- Therapeutic Drug Monitoring and Drug Quantification
- Pharmacokinetics and Pharmacodynamics of the Drugs
- Toxicogenomics Challenges and Applications
- Pharmacovigilance and Drug Safety
- Entrepreneurs Investment Meet
- Conducts of Clinical Trials
- Pre-Clinical Research
- Clinical Study Designs
- Innovations in Clinical Trials
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | August 22-24, 2016 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | Day 2 | Day 3 |
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Clinical Toxicology
- Journal of Pharmacogenomics & Pharmacoproteomics
- Journal of Drug Metabolism & Toxicology
Abstracts will be provided with Digital Object Identifier by